HARD
NEET
IMPORTANT
Earn 100
Which of the following plot is in accordance with the arrhenius equation : -
(a)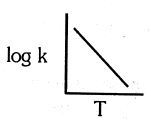

(b)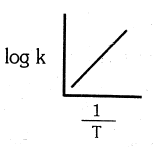

(c)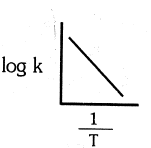

(d)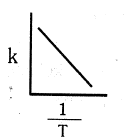

88.89% studentsanswered this correctly

Important Questions on Chemical Kinetics
EASY
NEET
IMPORTANT
The rate of reaction increases by the increase of temperature because:
EASY
NEET
IMPORTANT
For a certain gaseous reaction rise of temperature from to doubles the rate of reaction. What is the value of activation energy : -
MEDIUM
NEET
IMPORTANT
The activation energy for the forward reaction is and is . The activation energy for the backward reaction is:
MEDIUM
NEET
IMPORTANT
For producing the effective collisions, the colliding molecules must possess:
HARD
NEET
IMPORTANT
The half life for a reaction is - - - - of temperature:-
MEDIUM
NEET
IMPORTANT
For the reaction,which of the following does not express the reaction rate?
MEDIUM
NEET
IMPORTANT
Consider the reaction .
The equality relationship between and is:
HARD
NEET
IMPORTANT
The rate constants and for two different reactions are and , respectively. The temperature at which is:
