HARD
Earn 100
Which of the following shapes of is more stable and why?
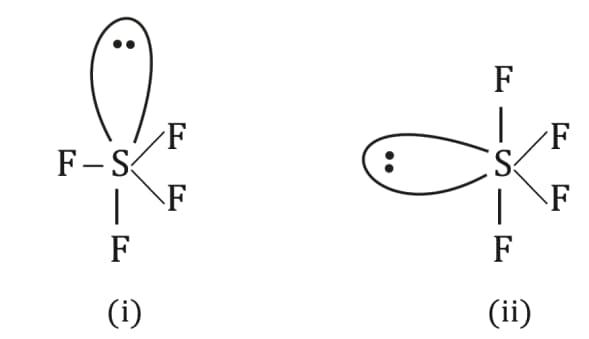
(a)(i), due to 3 lp-bp repulsion at
(b)(ii), due to 2 lp-bp repulsion
(c)Both are equally stable due to 2 lp-bp repulsions
(d)Both are unstable since has tetrahedral shape
100% studentsanswered this correctly
Important Questions on Chemical Bonding and Molecular Structure
MEDIUM
The correct statement about and is:
MEDIUM
The group of molecules having identical shape is:
HARD
Complexes of metals and have ideal square pyramidal and trigonal bipyramidal geometries, respectively. The sum of the and angles in the two complexes is_______
MEDIUM
If molecule is a polar molecule, a possible geometry of is :
EASY
The correct geometry and hybridization for are
HARD
The shape/structure of and , respectively are :
EASY
The shape of by VSEPR theory is:
EASY
Which of the following statements is false:
MEDIUM
Which of the following pairs of ions is isoelectronic and isostructural?
MEDIUM
The numbers of lone pair(s) on are respectively,
MEDIUM
The molecular geometry of is octahedral. What is the geometry of (including lone pair(s) of electrons, if (any)?
MEDIUM
In which of the following pairs, both the species are not isostructural?
HARD
The incorrect statement about carbonate ion is,
MEDIUM
The correct statement among the following is:
EASY
The geometry of molecule is
EASY
Bond angle in is more than that of . This is because
EASY
Incorrectly matched pair is :
EASY
The and bond angles (in degrees) in methane, ammonia and water are respectively, closest to-
MEDIUM
Among the following, which one is a wrong statement?
EASY
Predict the correct order among the following:

