MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
Which of the following statement(s) about the Arrhenius equation is/are correct?
(a)The pre-exponential factor becomes equal to the rate constant of the reaction at extremely high temperature.
(b)When the activation energy of the reaction is zero, the rate becomes independent of temperature.
(c)The term represents the fraction of the molecules having energy in excess of threshold value.
(d)On raising temperature, rate constant of the reaction of greater activation energy increases less rapidly than that of the reaction of smaller activation energy.
50% studentsanswered this correctly

Important Questions on Chemical Kinetics
HARD
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
Which of the following statements are true regarding the plot shown in the given diagram?
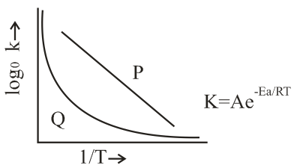
EASY
JEE Main/Advance
IMPORTANT
If the rate of reaction is given by: Rate
Which statements are correct?
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
