MEDIUM
NEET
IMPORTANT
Earn 100
Which of the following statement is true according to the kinetic theory of gases?
(a)The collision between two molecules is inelastic and the time between two collisions is less than the time taken during the collision.
(b)There is a force of attraction between the molecules.
(c)All the molecules of a gas move with same velocity.
(d)The average of the distances travelled between two successive collisions is mean free path.
43.48% studentsanswered this correctly

Important Questions on Kinetic Theory of Gases
EASY
NEET
IMPORTANT
At temperature, the kinetic energy of an ideal gas is . If the temperature is increased to , then kinetic energy would be
EASY
NEET
IMPORTANT
A diatomic molecule has
MEDIUM
NEET
IMPORTANT
A given mass of an ideal gas is at pressure $P$ and absolute temperature . The isothermal bulk modulus of gas is given by
MEDIUM
NEET
IMPORTANT
The pressure-temperature relationship for an ideal gas undergoing adiabatic change is
EASY
NEET
IMPORTANT
Two balloons are filled one with pure gas and other with air respectively. If the pressure and temperature of these balloons are same, then the number of molecules per unit volume is
EASY
NEET
IMPORTANT
At , the value of the density of a fixed mass of an ideal gas divided by its pressure is . At , this ratio is
EASY
NEET
IMPORTANT
Degree of freedom for polyatomic gas:-
EASY
NEET
IMPORTANT
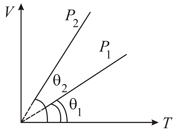
In the given (V-T) diagram, what is the relation between pressure P1 and P2?