HARD
Earn 100
Which of the following statements is/ are not correct for following compounds ?
(I) SCl2(OCH3)2 and (II) SF2(OCH3)2
(a)-OCH3 groups in both cases occupy the same position
(b)Cl - atoms occupy equatorial position in case of (I) and F - atoms occupy equatorial position in case of (II)
(c)Cl - atoms occupy axial position in case of (I) & F - atoms occupy equatorial position in case of (II)
(d)Cl - and F - atoms occupy either axial or equatorial position in case of (I) and (II)
50% studentsanswered this correctly
Important Questions on Chemical Bonding and Molecular Structure
MEDIUM
MEDIUM
EASY
How many (i) hybridised carbon atoms and (ii) bonds are present in the following compound?
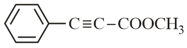
MEDIUM
MEDIUM
EASY
EASY
MEDIUM
EASY
| Column I | Column II |
| a. | (i) T-shape |
| b. | (ii) Pentagonal bipyramidal |
| c. | (iii) Linear |
| d. | (iv) Square – pyramidal |
| (v) Tetrahedral |
MEDIUM
EASY
EASY
(i)
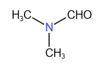 (ii)
(ii) 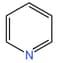
(iii) (iv)
MEDIUM
MEDIUM
EASY
EASY
EASY
HARD
MEDIUM
EASY

