EASY
Earn 100
Which one is an ore of sodium?
(a)Sylvine
(b)Siderite
(c)Spodumene
(d)Soda ash
50% studentsanswered this correctly
Important Questions on Principles of Metallurgy
MEDIUM
Which of the following statements are correct for the above chemical reaction?
(i) Lead is reduced.
(ii) Carbon di oxide is oxidised.
(iii) Carbon is oxidised.
(iv) Lead oxide is reduced
EASY
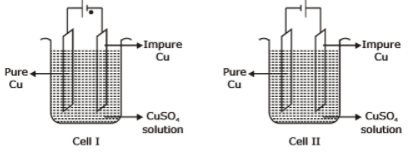
Consider the electrochemical cells(I and II) shown in the following figures and select the correct statement about these cells
EASY
MEDIUM
( are the atomic masses)
How much of Iron, we can get if 54 kg of Aluminium is used?
HARD
Give one word or a phrase for the following statement:
- Electrode used as a cathode in electrorefining of impure copper.
EASY
During electrolytic production of aluminium, the carbon anodes are replaced from time to time because
EASY
EASY
MEDIUM
MEDIUM
EASY
MEDIUM
MEDIUM
MEDIUM
MEDIUM
HARD
MEDIUM
MEDIUM
MEDIUM
MEDIUM

