MEDIUM
JEE Main
IMPORTANT
Earn 100
Which one of the following given graphs represents the variation of rate constant with temperature for an endothermic reaction?
(a)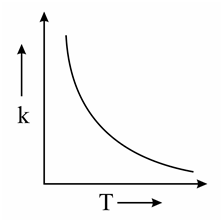

(b)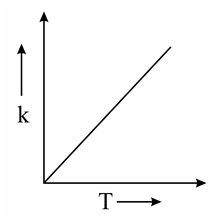

(c)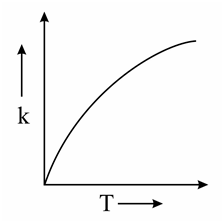

(d)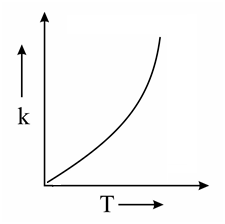

53.66% studentsanswered this correctly

Important Questions on Chemical Kinetics
MEDIUM
JEE Main
IMPORTANT
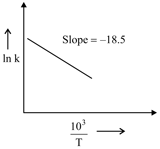
The rate constants for decomposition of acetaldehyde have been measured over the temperature range . The data has been analysed by plotting vs graph. The value of activation energy for the reaction is . (Nearest integer) (Given : )
MEDIUM
JEE Main
IMPORTANT
For a first order reaction, the time required for completion of reaction is '' times the half life of the reaction. The value of '' is
(Given: and )
HARD
JEE Main
IMPORTANT
For a given chemical reaction . Concentration of changes from to in Rate of appearance of is times the rate of disappearance of which is twice the rate of disappearance . The rate of appearance of has been experimentally determined to be Therefore the rate of reaction is____ (Nearest Integer)
HARD
JEE Main
IMPORTANT
At , the half life for the decomposition of a sample of a gaseous compound initially at was . When the pressure was , the half life was found to be . The order of the reaction is - [integer answer]
MEDIUM
JEE Main
IMPORTANT
Catalyst A reduces the activation energy for a reaction by at . The ratio of rate constants, is . The value of is____[nearest integer] [Assume that the pre-exponential factor is same in both the cases. Given ]
MEDIUM
JEE Main
IMPORTANT
A flask is filled with equal moles of and . The half lives of and are and respectively and are independent of the initial concentration. The time required for the concentration of to be four times that of is____.
(Given : )
MEDIUM
JEE Main
IMPORTANT
The rate constant for a first order reaction is given by the following equation :
The Activation energy for the reaction is given by . (In Nearest integer) (Given : )
MEDIUM
JEE Main
IMPORTANT
It has been found that for a chemical reaction with rise in temperature by the rate constant gets doubled. Assuming a reaction to be occurring at , the value of activation energy is found to be . [nearest integer] (Given )
