HARD
JEE Main
IMPORTANT
Earn 100
Which one of the following graphs is not correct for ideal gas?
Density, Pressure, Temperature
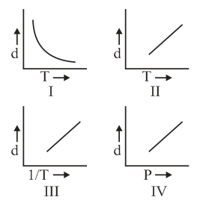

(a)I
(b)II
(c)IV
(d)III
38.36% studentsanswered this correctly

Important Questions on States of Matter: Gases and Liquids
EASY
JEE Main
IMPORTANT
Sulphur dioxide and oxygen were allowed to diffuse through a porous partition. of diffuses through the porous partition in seconds. The volume of in which diffuses under the similar condition in seconds will be (atomic mass of sulphur);
MEDIUM
JEE Main
IMPORTANT
Assuming ideal gas behavior, the ratio of density of ammonia to that of hydrogen chloride at same temperature and pressure is: (Atomic weight of Cl is 35.5 u)
MEDIUM
JEE Main
IMPORTANT
An open vessel at is heated until two fifth of the air (assumed as an ideal gas) in it has escaped from the vessel. Assuming that the volume of the vessel remains constant, the temperature to which the vessel has been heated is:
MEDIUM
JEE Main
IMPORTANT
The volume of gas is twice than that of gas . The compressibility factor of gas is thrice than that of gas at same temperature. What are the pressures of the gases for equal number of moles?
MEDIUM
JEE Main
IMPORTANT
When does a gas deviate the most from its ideal behavior?
MEDIUM
JEE Main
IMPORTANT
Points and in the following plot respectively correspond to ( most probable velocity)
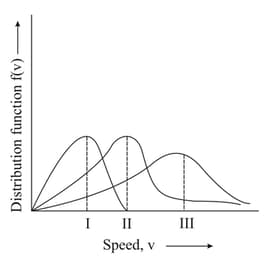
EASY
JEE Main
IMPORTANT
Consider the following table:
| Gas | ||
|---|---|---|
| A | ||
| B | ||
| C | ||
| D |
and are vander Waals constants. The correct statement about the gases is:
EASY
JEE Main
IMPORTANT
Initially, the root-mean-square (RMS) velocity of molecules at certain temperature is . If this temperature is doubled and all the nitrogen molecules dissociate into nitrogen atoms, then the new RMS velocity will be:
