Which one of the following is a correct electronic configuration of sodium ?
Important Questions on Structure of The Atom
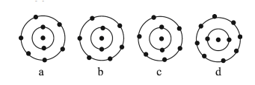
The schematic atomic structure of four elements is given below. Observe and choose the right statement.
Match the following :
List -I :
(a) Frequency of distribution of the emitted radiation from a black body
(b) Spin quantum numbers(ms)
(c) Angular Momentum
(d) All orbital have equal energy
List - II :
(i) degeneracy
(ii) temperature dependent
(iii) vector quantity
(iv) mass times velocity times radius
Codes:
An isoelectronic species are
Nitrogen (atomic number ) and phosphorus (atomic number ) belong to group of the Periodic Table. Write the electronic configuration of these two elements. Which one of these will be more electronegative? Why?
What do you understand by the term electronic configuration? State Bohr-Bury's scheme of electronic configuration.

