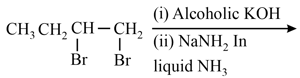HARD
Earn 100
Which one of the following statements about the physical properties of alkenes is true ?
(a)Alkenes have a lower density than water.
(b)They density of alkenes increases with the number of atoms.
(c)Cis isomerism do not affect density of alkene.
(d)Boiling point and densities are not correlated in case of alkenes.
14.29% studentsanswered this correctly
Important Questions on Hydrocarbons
HARD
EASY
HARD
What is the major product expected from the following reaction?
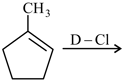
Where D is an isotope of hydrogen.
MEDIUM
MEDIUM
MEDIUM
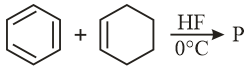
The major product of the following reaction is:
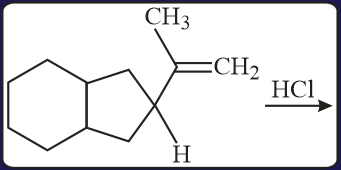
EASY
HARD
MEDIUM
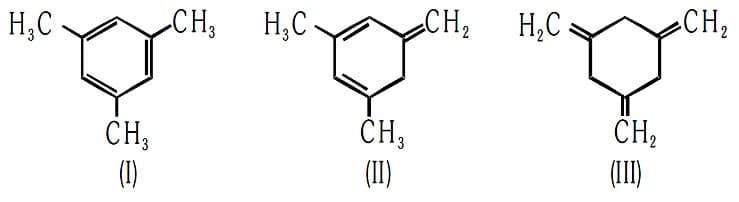
The enthalpy of hydrogenation of these compounds will be in the order as:
HARD
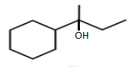 ?
?MEDIUM
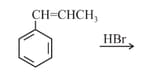
MEDIUM

HARD
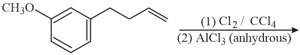
The major product in the following conversion is
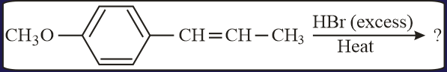
EASY