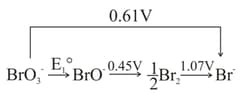MEDIUM
Earn 100
Which statement is not true regarding the relationship of the free-energy change and the cell potential of a galvanic cell?
(a)When the cell potential is used to determine the free-energy change, the energy in joules is obtained.
(b)If the free-energy change indicates a spontaneous reaction,
then the cell potential has a negative value.
(c)The free-energy change and the cell potential of a galvanic
cell are quantitative measures of the driving force of
a chemical reaction.
(d)Both the free-energy change and the cell potential are
dependent on the composition of the reaction mixture.
50% studentsanswered this correctly
Important Questions on Electrochemistry
EASY
MEDIUM
at is approximately:
EASY
MEDIUM
MEDIUM
MEDIUM
The standard emf of the cell and equilibrium constant of the following reaction of
EASY
EASY
MEDIUM
The emf of the following cell is at .
The standard free energy change for the cell reaction is________
EASY
MEDIUM
is
EASY
MEDIUM
The standard e.m.f. of the cell, in which the cell reaction is is at and at . The enthalpy change of the reaction at is
MEDIUM
MEDIUM
MEDIUM
For the cell reaction
at The standard Gibbs energy of the cell reaction is:
[Given that Faraday constant ]
EASY
Given that:
HARD
The standard electrode potential and its temperature coefficient for a cell are and at , respectively. The reaction is . The standard reaction enthalpy at in is
Use and