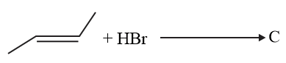Which will have the highest boiling point
Important Questions on Haloalkanes and Haloarenes
Identify the correct order for the given property for following compounds
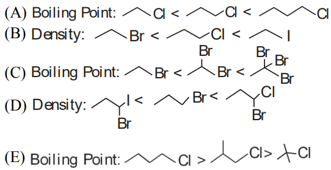
Choose the correct answer from the option given below :-
The major product of the following reaction is:
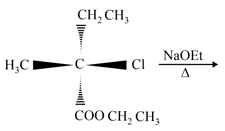
The major product of the following reaction is:
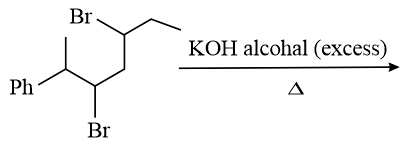
Give reasons:
Haloalkanes easily dissolve in organic solvents.
Give reasons:
C — Cl bond length in chlorobenzene is shorter than C — Cl bond length in chloromethane.
The correct decreasing order of densities of the following compounds is :
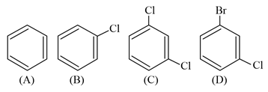
a.
b.
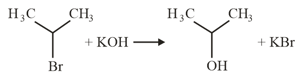
c.
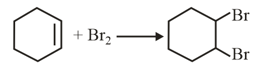
Which of the following statements is correct?
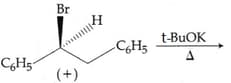
The major product of the following reaction is:
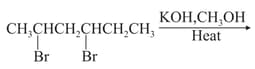
Give reason:
n-Butyl bromide has higher boiling point than t-Butyl bromide.
Arrange the following compounds in the decreasing order of boiling points:
The increasing order of the boiling points of the major products and of the following reactions will be :
(a)
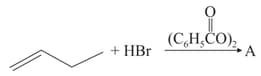
(b)
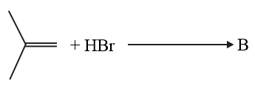
(c)