HARD
Earn 100
Which would undergo reaction faster in the following pair and why?
Ethyl bromide and tertiary butyl bromide
Important Questions on Halogen Derivatives
MEDIUM
The increasing order of reactivity of the following compounds towards reaction with alkyl halides directly is:
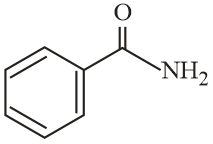
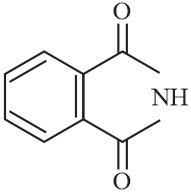
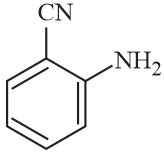
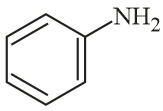
EASY
MEDIUM
MEDIUM
(I)
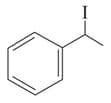
(II)
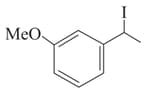
(III)
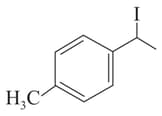
(IV)
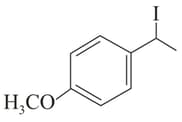
MEDIUM
MEDIUM
MEDIUM
(1)
(2)
(3)
is
MEDIUM
EASY
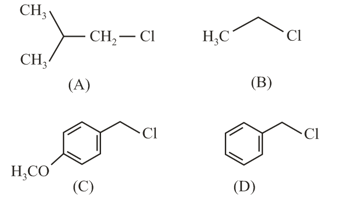
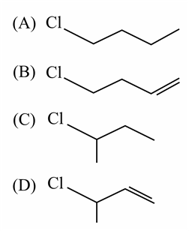
This reaction will be the fastest in
EASY
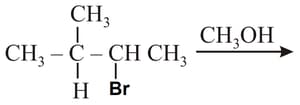
The reagent required for the following two step transformation are
MEDIUM
MEDIUM
MEDIUM
HARD
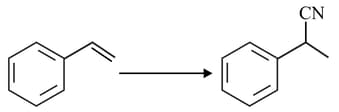
The major product of the following reaction is:
MEDIUM
Assertion : Vinyl halides do not undergo nucleophilic substitution easily.
Reason : Even though the intermediate carbocation is stabilized by loosely held electrons, the cleavage is difficult because of the strong bonding.
MEDIUM
The mechanism of Sn1 reaction is given as:
A student writes general characteristics based on the given mechanism as :
(a) The reaction is favoured by weak nucleophiles.
(b) would be easily formed if the substituents are bulky
(c) The reaction is accompanied by recemization
(d) The reaction is favoured by non-polar solvents.
Which observations are correct?
HARD
EASY
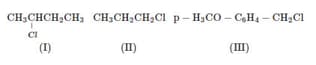
MEDIUM
CH3Cl, CH3CH2Cl, (CH3)2CHCl and (CH3)3CCl is :
EASY
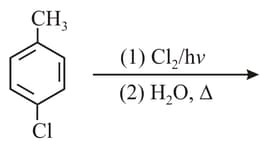
In the reaction


