EASY
12th CBSE
IMPORTANT
Earn 100
Why can’t molecularity of any reaction be equal to zero?

Important Questions on Chemical Kinetics
EASY
12th CBSE
IMPORTANT
EASY
12th CBSE
IMPORTANT
MEDIUM
12th CBSE
IMPORTANT
Match the graph given in Column I with the order of reaction given in Column II. More than one item in Column I may link to the same item of Column II.
| Column I | Column II |
(i) 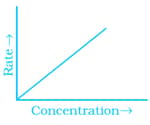 |
|
(ii) 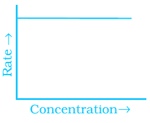 |
(a) Ist order |
(iii) 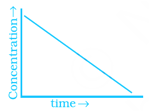 |
(b) Zero order |
(iv) 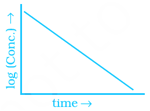 |
MEDIUM
12th CBSE
IMPORTANT
Match the items of Column I and Column II.
| Column I | Column II |
| (i) Diamond | (a) short interval of time |
| (ii) Instantaneous rate | (b) ordinarily rate of conversion is imperceptible |
| (iii) Average rate | (c) long duration of time |
MEDIUM
12th CBSE
IMPORTANT
Match the items of Column I and Column II.
| Column I | Column II |
| (i) Mathematical expression for the rate of reaction | (a) rate constant |
| (ii) Rate of reaction for the zero-order reaction is equal to | (b) rate law |
| (iii) Units of rate constant for the zero-order reaction is same as that of | (c) order of slowest step |
| (iv) Order of a complex reaction is determined by | (d) rate of a reaction |
HARD
12th CBSE
IMPORTANT
MEDIUM
12th CBSE
IMPORTANT
MEDIUM
12th CBSE
IMPORTANT
