MEDIUM
MYP:4-5
IMPORTANT
Earn 100
Why is the density of ice lower than that of liquid water? Give an example of where this can be observed on our planet.

Important Questions on Form
HARD
MYP:4-5
IMPORTANT
HARD
MYP:4-5
IMPORTANT
HARD
MYP:4-5
IMPORTANT
Compare these values of enthalpies of fusion and vaporization. Explain why the enthalpy of vaporization for a substance is greater than its enthalpy of fusion.
| Enthalpy of fusion | Enthalpy of vaporization | |
| Water | ||
| Carbon dioxide | ||
| Lead |
MEDIUM
MYP:4-5
IMPORTANT
MEDIUM
MYP:4-5
IMPORTANT
MEDIUM
MYP:4-5
IMPORTANT
MEDIUM
MYP:4-5
IMPORTANT
HARD
MYP:4-5
IMPORTANT
The boiling point of water is at an atmospheric pressure of atmosphere or , a typical level at sea level. At higher altitudes the air pressure decreases and the boiling point increases.
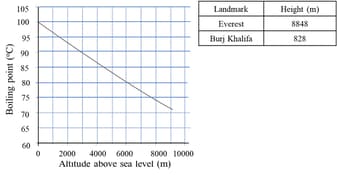
Study the graph and the table above. Use the information to calculate the percentage increase of the boiling point from the summit of Everest to the top of the Burj Khalifa.
