MEDIUM
Earn 100
With the help of a graph explain why it is not possible to determine for a weak electrolyte by extrapolating the concentration-molar conductance curve as for strong electrolyte.
Important Questions on Electrochemistry
MEDIUM
MEDIUM
MEDIUM
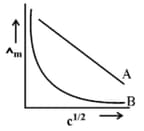
In the plot of molar conductivity vs square root of concentration , following curves are obtained for two electrolytes A and B :
Answer the following :
Predict the nature of electrolytes A and B.
EASY
EASY
HARD
MEDIUM
EASY
MEDIUM
MEDIUM
Given below are two statements:
Statement I : The limiting molar conductivity of (strong electrolyte) is higher compared to that of $\mathrm{CH}_{3} \mathrm{COOH}$ (weak electrolyte).
Statement II : Molar conductivity decreases with decrease in concentration of electrolyte.
In the light of the above statements, choose the most appropriate answer from the options given below:
EASY
MEDIUM
Conductivity of dilute hydrochloric acid is greater than that of acetic acid.
HARD
MEDIUM
HARD
HARD
Define the following and write the formula and unit :
Equivalent conductivity
MEDIUM
MEDIUM
Match List - I with List - II :
| List - I (Parameter) |
List - II (Unit) | ||
| (a) | Cell constant | (i) | |
| (b) | Molar conductivity | (ii) | Dimensionless |
| (c) | Conductivity | (iii) | |
| (d) | Degree of dissociation of electrolyte | (iv) |
HARD
HARD