EASY
Earn 100
With the increase in temperature, the kinetic energy of the particle _____.
(a)Increases
(b)Decreases
(c)Remains constant
(d)None of the above
68.42% studentsanswered this correctly
Important Questions on Matter in our Surroundings
MEDIUM
(R = 8.314 J/mol K) (ln7.5 = 2.01)
MEDIUM
The combination of plots which does not represent isothermal expansion of an ideal gas is




HARD

The above diagram represents the thermodynamic cycle of an engine, operating with an ideal mono-atomic gas. The amount of heat, extracted from the source in a single cycle, is:
EASY
MEDIUM
EASY
HARD

The correct option(s) is (are)
MEDIUM
EASY
(Latent heat of ice is and )
MEDIUM

MEDIUM
[Heat of fusion of ice ; Specific heat of water ]
MEDIUM
Which of the following statements are true?
I. On heating the kinetic energy of particles in solids does not change because they have a fixed position.
II. Sublimation is the change of gaseous state directly to solid state without going through liquid state and vice versa.
III. The movement of particles from an area of higher concentration to lower concentration is called diffusion.
IV. The rate of evaporation is not affected by increasing the temperature.
EASY
MEDIUM
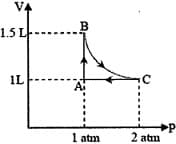
EASY
HARD
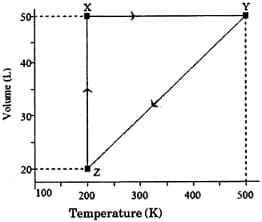
The pressure of the gas (in atm) at and respectively, are
MEDIUM
HARD
EASY
MEDIUM

