MEDIUM
AS and A Level
IMPORTANT
Earn 100
Write a balanced equation for the oxidation of butan--ol to butanone, using to represent an oxygen atom from the oxidising agent.

Important Questions on Carbonyl Compounds
MEDIUM
AS and A Level
IMPORTANT
What do you observe in the reaction vessel if the oxidising agent i.e. potassium dichromate (VI) used in the oxidation of ethanol to ethanal reaction is acidified with dilute sulphuric acid, and the reaction mixture is heated?
MEDIUM
AS and A Level
IMPORTANT
The butanone does not have to be distilled off as it forms in the reaction vessel because _____.
HARD
AS and A Level
IMPORTANT
Write a balanced equation for the reaction that takes place when proapanal is warmed with an aqueous alkaline solution of sodium tetrahydridoborate, using the symbol to represent a hydrogen atom from the reducing agent.
HARD
AS and A Level
IMPORTANT
Name the product formed in the reduction reaction if pentan--one is added to lithium tetrahydridoaluminate in dry ether.
MEDIUM
AS and A Level
IMPORTANT
Name the organic product that would be formed in the nucleophilic addition of to ethanal.
HARD
AS and A Level
IMPORTANT
Name the organic product that would be formed in the nucleophilic addition of to propanone.
HARD
AS and A Level
IMPORTANT
Use diagram and curly arrows to describe the mechanism of the reaction of nucleophilic addition of to ethanal.
HARD
AS and A Level
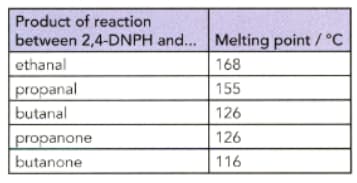
IMPORTANT
The melting points of the derivatives of the reaction between -DNPH and various aldehydes and ketones are shown in the table.
Describe your observations when each of the carbonyl compounds in the table is mixed with -DNPH.