Write a balanced equation for the reaction of 2-bromopropane with ethanolic sodium hydroxide.

Important Questions on Halogenoalkanes
What type of reaction best describes the heating of chloroethane with ethanolic sodium hydroxide solution?
Which one of the following is a tertiary halogenoalkane?

Which one of the following will form a carbocation in the mechanism of nucleophilic substitution?

When a solution of potassium cyanide in ethanol is heated under reflux with 1-chloropropane, what is the organic product of the reaction called?

1-bromobutane will undergo reactions when heated, as shown by reactions A and B.
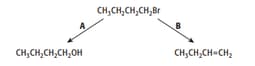
For reactions A and B give the reagents used in each case.
1-bromobutane will undergo reactions when heated, as shown by reactions A and B.
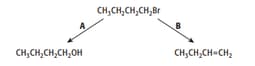
Reaction A was repeated using 1-iodobutane instead of 1-bromobutane. Explain any difference in the rate of reaction observed.
1-bromobutane will undergo reactions when heated, as shown by reactions A and B.
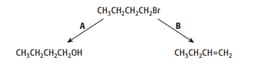
Reaction A was repeated with 2-bromo-2-methylpropane instead of 1-bromobutane.
The mechanism of the reaction with 2-bromo-2-methylpropane differs from the mechanism of reaction A. Describe how the mechanisms differ.
1-bromobutane will undergo reactions when heated, as shown by reactions A and B.
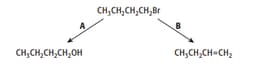
If reaction B was repeated with 2-bromobutane, name the other organic products that can form as well as the product shown above.
