Write an example for homogeneous equilibrium reaction.
Important Questions on Equilibrium
What is the effect of pressure in this reversible reaction? Explain.
Write any one activity to increase the red colour in the following reaction.
What is the total number of moles of reactants and products in the above reaction?
Graph of a reversible process;
is given. Analyse the graph and answer the following question.
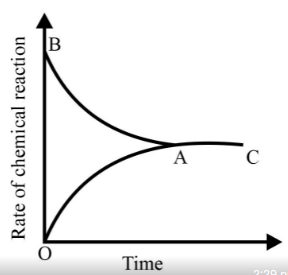
Identify the part of the graph which represents the forward reaction
[ OA, BA, AC]
What happens to the rate of forward reaction of the equilibrium,
during the following situations?
(a) increase in temperature
(b) is removed
(c) pressure is decreased
For this equilibrium, the correct statement(s) is/are
Graph of a reversible process;
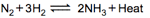
is given. Analyse the graph and answer the following question.
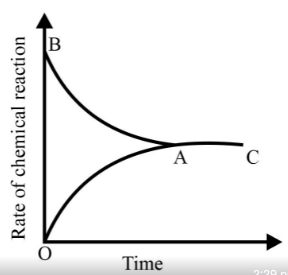
Identify the part of the graph which represents the equilibrium state?
Graph of a reversible process,
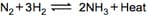
is given. Analyse the graph and answer the following question.
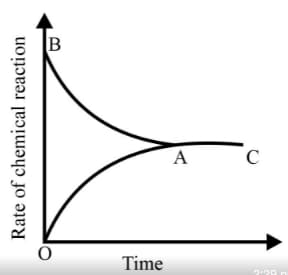
From the given statements, select the correct ones regarding chemical equilibrium.
(i0 The chemical equilibrium is 'static' at the molecular level.
(ii) Both reactants and products co-exist.
(iii) The rates of forward reaction and backward reactions are equal.
(iv) Chemical equilibrium is attained in an open system.
Which of the following statement is correct about chemical equilibrium?
(i) At equilibrium both the reactants and products coexist.
(ii) At equilibrium rate of forward reaction is greater than the rate of backward reaction.
At equilibrium mixture for the reaction
2H2S ⇌2H2 + S2
had 1 mole of H2S, 0.2 mole of H2, and 0.8 mole of S2 in a
2 litre flask. The value of Kc in mol L- is

