EASY
AS and A Level
IMPORTANT
Earn 100
Write ionic equations for the reaction in aqueous solution between sodium hydroxide and nitric acid.

Important Questions on Equilibria
EASY
AS and A Level
IMPORTANT
Write ionic equations for the reaction in aqueous solution between potassium hydroxide and hydrochloric acid.
EASY
AS and A Level
IMPORTANT
Identify which reactants are acids and which are bases in the following reactions: .
EASY
AS and A Level
IMPORTANT
Identify which reactants are acids and which are bases in the following reactions: .
EASY
AS and A Level
IMPORTANT
Identify the acid and the base on the right-hand side of these equilibria: .
EASY
AS and A Level
IMPORTANT
Identify the acid and the base on the right-hand side of these equilibria: .
EASY
AS and A Level
IMPORTANT
Hydrazine, , is a weak base. Write a chemical equation to show the equilibrium reaction of hydrazine with water.
EASY
AS and A Level
IMPORTANT
Hydrazine, , is a weak base. State the relative concentrations (high or low) of the , molecules and the products.
EASY
AS and A Level
IMPORTANT
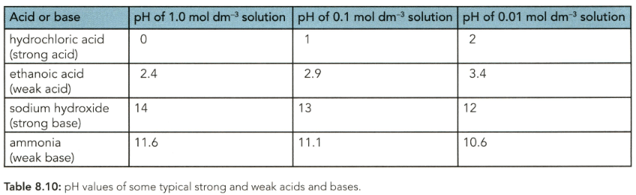
The of a solution depends on the hydrogen ion (hydroxonium ion) concentration. Which concentration of ethanoic acid in Table has the highest concentration of hydrogen ions in solution?