EASY
JEE Main/Advance
IMPORTANT
Earn 100
Write isomeric amines of the formula .

Important Questions on Amines
EASY
JEE Main/Advance
IMPORTANT
The two amines shown differ by a factor of about in their ionisation constants. Which is stronger base? Explain :
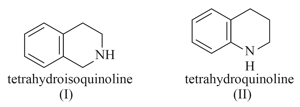
MEDIUM
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
