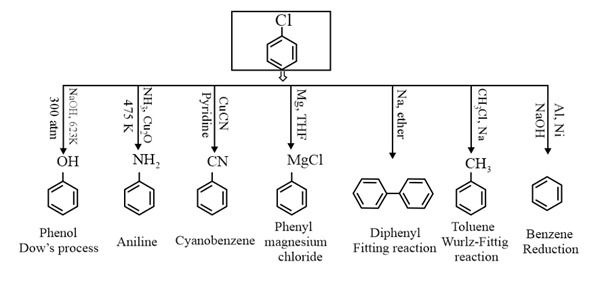
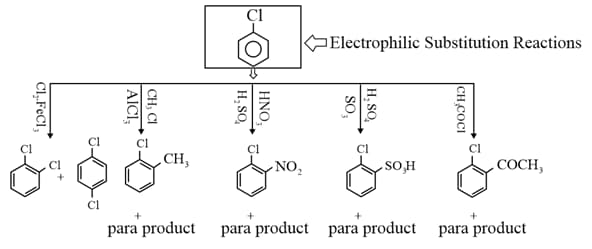
Write short note on the following.
Darzen's process

Important Points to Remember in Chapter -1 - Haloalkanes and Haloarenes from Tamil Nadu Board Chemistry Standard 11 Vol II Solutions
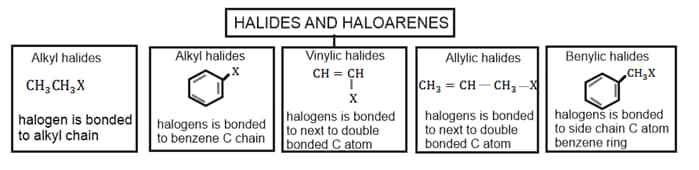
1. Halides and Haloarenes:
2. Preparation of Haloalkanes:
(i) From Alcohols:
(ii) From Hydrocarbons:
(iii) From alkanes by free radical halogenation
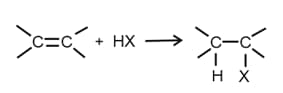
(iv) From Alkenes by addition of hydrogen halides -
(v) From Alkenes by addition of Halogens
(vi) By Halogen exchange:
(a) Finkelstein reaction:
(b) Swarts reaction:
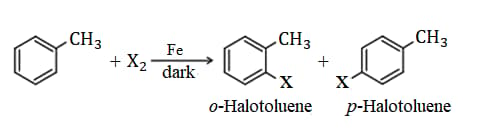
3. Preparation of Haloarenes:
(i) From hydrocarbons by electrophilic substitution
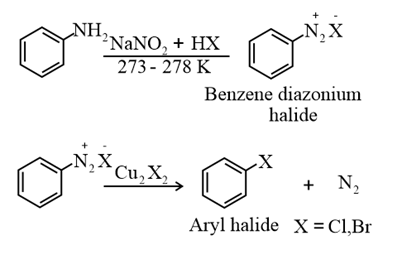
(ii) From amines by Sandmeyer’s reaction
4. Nucleophilic substitution reactions
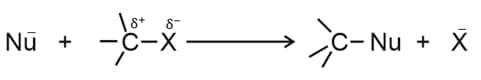
(i) The reactivity of alkyl halides towards mechanism is: methyl .
(ii) The mechanism occurs through the formation of carbonation. The order of reactivity is: methyl
5. Reactions with metals
Wurtz reaction:
6. Reduction:
7. Elimination reactions:
8. Reaction of Haloarenes: