MEDIUM
Earn 100
Write the balanced equation for the laboratory preparation of methane.
Important Questions on Organic Chemistry
MEDIUM
MEDIUM
MEDIUM
EASY
MEDIUM
HARD
State one relevant reason for the following:
- Soda-lime is preferred to sodium hydroxide in the laboratory preparation of methane.
EASY
Wurtz reaction
Reduction of alkyl halides with zinc and dil.
Kolbe's electrolysis
Catalytic hydrogenation of alkenes
MEDIUM
Hydrogenation of cyclohexene and benzene take place as shown below. The resonance energy of benzene is
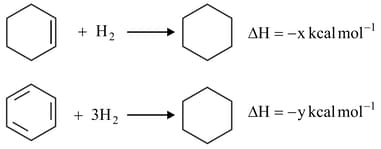
The relationship between and is
EASY
MEDIUM
MEDIUM
HARD
Write a balanced chemical equation for the following:
- Producing ethane from bromo-ethane using couple in alcohol.
MEDIUM
What is hydrogenation? What is its industrial application?
MEDIUM
HARD
MEDIUM

