HARD
Earn 100
Write the following statement of about RESONANCE are true or false.
There is no equilibrium between the canonical forms.
(a)True
(b)False
50% studentsanswered this correctly
Important Questions on Chemical Bonding and Molecular Structure
MEDIUM
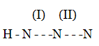
The above compound represents hydrogen azide, the bond orders of bonds and are:
MEDIUM
[Resonance energy of benzene
Enthalpy of hydrogenation of cyclohexene ]
MEDIUM
Resonance in carbonate ion is
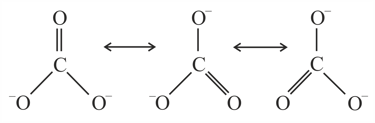
Which of the following is true?
EASY
MEDIUM
MEDIUM
EASY
EASY
MEDIUM
MEDIUM
The molecule/molecules that has/have delocalised lone pair(s) of electrons is/are
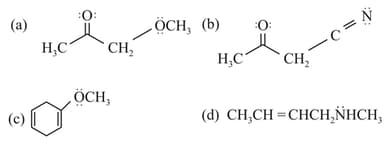
MEDIUM
MEDIUM
Find out the correct order of ionic character in the following molecules
HARD
MEDIUM
MEDIUM
EASY
MEDIUM
Give a reason for the following:
Ionic compounds have a high melting point.
MEDIUM
EASY
EASY

