Write the formation process of micelles.
Important Questions on Carbon and its Compounds
Write short note on following-
- Detergent
Detergents are also called surface-active agents (surfactants). These have two distinct parts: one hydrophilic spherical part and another hydrophobic long tail made of carbons chain. Two experiment ‘A’ and ‘B’ were carried out. In experiment ‘A’, surfactant was added in a beaker containing water. In experiment ‘B’, surfactant was added in a beaker containing hexane.
Following are possible results in these experiments.
I. In experiment ‘A’(see figure) ‘a’ micelle is formed, where hydrophobic part is inside the micelle and hydrophilic part is outside the micelle.
II. In experiment ‘B’(see figure ‘b’) micelle of reverse type is formed where hydrophilic part is inside the micelle and hydrophobic part is outside the micelle.
III. Micelle of reverse type is formed in experiment ‘A’.
IV. Micelles are large enough to scatter light in ‘A’
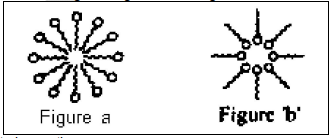
Correct observations are:
Name the structure shown in the figure. Label 1 and 2.
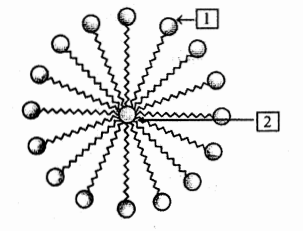
Explain the mechanism of the cleaning action of soaps.
Which of the following statements are false about soaps and detergents?
(i) Soaps are water-soluble while detergents are not.
(ii) Soaps are non-biodegradable while detergents are biodegradable.
(iii) Hardness of water is due to the presence of and salts that form scum with soap.
(iv) The polar group in soaps is .

