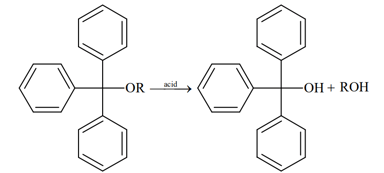
HARD
Earn 100
Write the mechanism of action of hydrogen bromide on tert-Butyl methyl ether.
Important Questions on Alcohols, Phenols and Ethers
MEDIUM
HARD
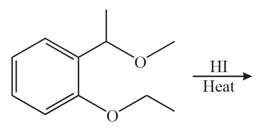
HARD
MEDIUM
HARD
MEDIUM
The major product in the following reaction is :
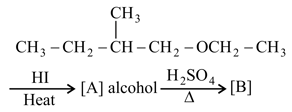
MEDIUM
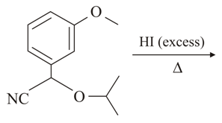
EASY
What are the products formed in the following reaction?
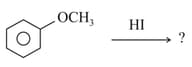
MEDIUM
EASY
MEDIUM
What is in the following change?
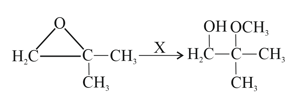
EASY
Write the structures of the main products in the following reactions:
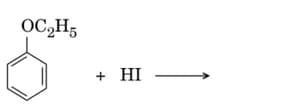
EASY
HARD
The major aromatic product in the following reaction sequence will be :
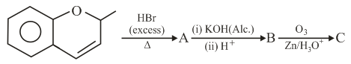
EASY
MEDIUM
MEDIUM
MEDIUM
MEDIUM
Anisole to p-bromoanisole
HARD