MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
Write the name of the following anions.

Important Questions on Chemical Bonding and Molecular Structure
EASY
JEE Main/Advance
IMPORTANT
The correct formula of aluminium perchlorate is _______.
EASY
JEE Main/Advance
IMPORTANT
Aluminium phosphide is:
EASY
JEE Main/Advance
IMPORTANT
Formula of Dioxygen diflouride is:
EASY
JEE Main/Advance
IMPORTANT
Silicon fluoride formula is ______.
EASY
JEE Main/Advance
IMPORTANT
With the help of Lewis dot structure, find the number of total covalent bonds in species.
(i), (ii) , (iii)
EASY
JEE Main/Advance
IMPORTANT
Indicate what is wrong with each of the following Lewis structures. Replace each with a more acceptable structure.
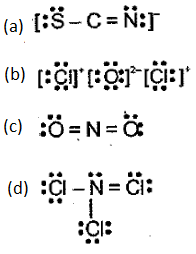
EASY
JEE Main/Advance
IMPORTANT
In how many of the following species, the central atoms have two lone pairs of electrons?
(i)
(ii)
(iii)
(iv)
(V)
(vi)
(vii)
(viii)
(ix)
EASY
JEE Main/Advance
IMPORTANT
How many compounds violate octet rule?
(i)
(ii)
(iii)
(iv)
(v)
(vi)
(vi) friii)
