MEDIUM
Earn 100
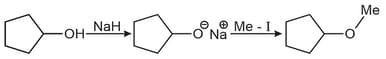
Write the reaction for the preparation of diethyl ether using dry silver oxide.
Important Questions on Alcohols, Phenols and Ethers
EASY
MEDIUM
How will you obtain anisole from phenol.
EASY
EASY
EASY
EASY
MEDIUM
| Column - I | Column - II | ||
|---|---|---|---|
| Williamson’s synthesis | in presence of pyridine | ||
| Nitrophenol | Unsymmetrical ether | ||
| Acetylation | Intermolecular hydrogen bonding |
MEDIUM
HARD
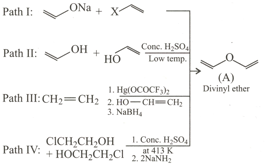
Which of the following paths is/are feasible for the preparation of ether (A) ?
EASY
The major product of the following reactions is
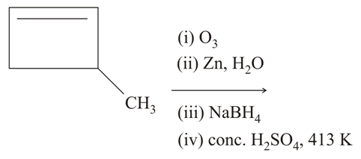
MEDIUM
MEDIUM
Identify the reagent for the given reaction:
MEDIUM
MEDIUM
EASY
EASY
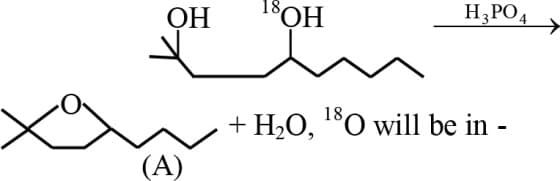
Identify the ether () formed from the below reaction:
MEDIUM
MEDIUM
HARD