HARD
Earn 100
Write the reaction of chromyl chloride with sodium hydroxide.
Important Questions on Inorganic Qualitative Analysis
HARD
Distinguish between the following pair of compounds using a reagent as a chemical test:
- Magnesium chloride and magnesium nitrate solution.
EASY
MEDIUM
side products
side products
side products
The sum of the total number of atoms in one molecule each of and is ________
MEDIUM
HARD
EASY
MEDIUM
MEDIUM
MEDIUM
HARD
HARD
HARD
EASY
HARD
brown fumes. Which is the correct statement regarding the above observation.
MEDIUM
Some reactions are given for salt(X).
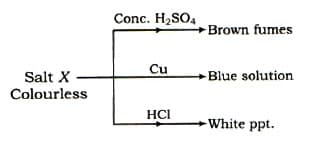
Which of the following salt can be satisfied all the conditions?
HARD
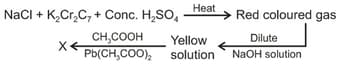
MEDIUM
HARD
HARD
EASY

