HARD
Earn 100
Write the statement for: Boyle's law
Important Questions on States of Matter
HARD
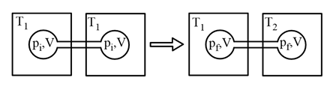
EASY
MEDIUM
MEDIUM
EASY
At constant pressure, the volume of a fixed mass of a gas varies as a function on temperature as shown in the graph
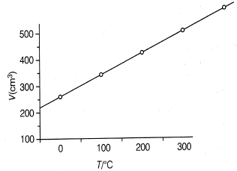
The volume of the gas at is larger than that at by a factor of
EASY
EASY
EASY
EASY
EASY
MEDIUM
MEDIUM
EASY
EASY
HARD
Which one of the following graphs is not correct for ideal gas?
Density, Pressure, Temperature
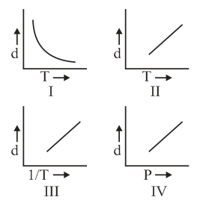
MEDIUM
HARD
MEDIUM
[Gas constant, ]
MEDIUM
MEDIUM
The volume of gas is twice than that of gas . The compressibility factor of gas is thrice than that of gas at same temperature. What are the pressures of the gases for equal number of moles?

