Zinc sulphate forms a colourless solution in water. Will you observe any colour on adding copper turning in it?
Important Questions on Sorting Materials into Groups
Which gas is usually liberated when an acid reacts with a metal? Illustrate with an example. How will you test for the presence of this gas?
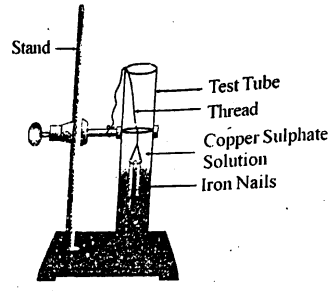
Write a chemical equation for the reaction taking place in the test tube. How does the colour of the solution change? What is the change in colour of iron nails?
What is the nature of oxides formed,
(a) when metals combine with oxygen?
(b) when non-metals combine with oxygen?
Complete the following reaction:
Write the conditions under which rusting takes place.
What is your observation in the following case:
Burning of magnesium in air.
Can copper displace zinc from its salt solution?
What happens when magnesium reacts with oxygen? What is the nature of the product?
Write your observation when a piece of sodium is dropped in water.

