EASY
JEE Main
IMPORTANT
Earn 100
moles of an ideal gas with constant volume heat capacity undergo an isobaric expansion by certain volume. The ratio of the work done in the process, to the heat supplied is:
(a)
(b)
(c)
(d)
83.54% studentsanswered this correctly

Important Questions on Thermodynamics
MEDIUM
JEE Main
IMPORTANT
A cylinder with fixed capacity of litre contains helium gas at STP. The amount of heat needed to raise the temperature of the gas by is:
[Given that
MEDIUM
JEE Main
IMPORTANT
When heat is supplied to a diatomic gas of rigid molecules, at constant volume its temperature increases by . The heat required to produce the same change in temperature, at a constant pressure is:
MEDIUM
JEE Main
IMPORTANT
Three Carnot engines operate in series between a heat source at a temperature and a heat sink at temperature (see figure). There are two other reservoirs at temperature and as shown, with The three engines are equally efficient if:
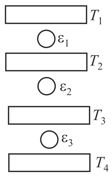
EASY
JEE Main
IMPORTANT
of a monoatomic gas is at a pressure of . The density of the gas is . What is the order of energy of the gas due to its thermal motion?
EASY
JEE Main
IMPORTANT
Half mole of an ideal monoatomic gas is heated at a constant pressure of from to . Work done by the gas isGas constant,)
MEDIUM
JEE Main
IMPORTANT
Two moles of helium gas is mixed with three moles of hydrogen molecules (taken to be rigid). What is the molar specific heat of mixture at constant volume?
MEDIUM
JEE Main
IMPORTANT
A sample of an ideal gas is taken through the cyclic process as shown in the figure. The change in the internal energy of the gas along the path is The gas absorbs of heat along the path and along the path . The work done by the gas along the path is:
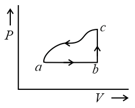
MEDIUM
JEE Main
IMPORTANT
A Carnot engine has an efficiency of When the temperature of the sink is reduced by , its efficiency is doubled. The temperatures of the source and the sink are, respectively,
