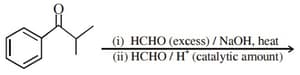HARD
Earn 100
amino benzaldehyde is heated along with acetone in dilute solution to produce the major organic product , which on treatment with catalytic amount of , produces another major organic product formed via intramolecular reaction. Let, the degree of unsaturation of be , the degree of unsaturation of be , the number of rings in be and the number of rings in be , then, find the value of
50% studentsanswered this correctly
Important Questions on Aldehydes, Ketones and Carboxylic Acids
HARD
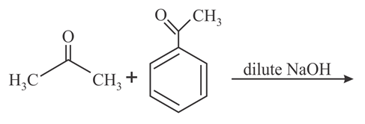
The major product obtained in the following reaction is:
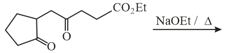
MEDIUM
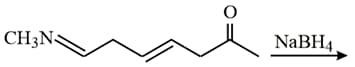
The major product formed in the following reaction is:
HARD
The major product of the following reaction is:
HARD
In the following reaction sequence, the amount of (in g) formed from moles of acetophenone is ____.
(Atomic weights in The yield (%) corresponding to the product in each step is given in the parenthesis)
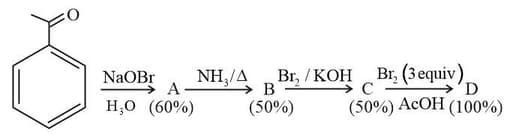
MEDIUM
The major product 'X' formed in the following reaction is:
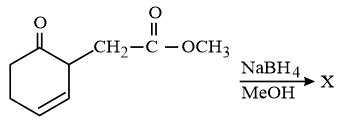
MEDIUM
The increasing order of the reactivity of the following with is:
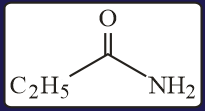
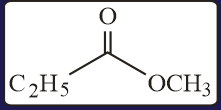
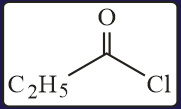
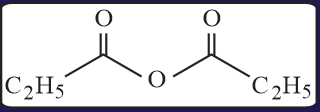
HARD
The test performed on compound and their inferences are:
| Test | Inference |
| - test | Coloured precipitate yellow |
| Iodoform test | Yellow precipitate |
| Azo-dye test | No dye formation |
Compound is:
HARD
Treatment of cyclopentanone
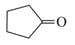 with methyl lithium gives which of the following species?
with methyl lithium gives which of the following species?EASY
In the following reaction
MEDIUM
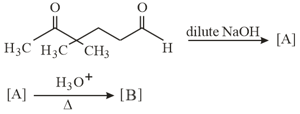
In the following reactions, products and are:
EASY
Reaction of a carbonyl compound with one of the following reagents involves nucleophilic addition followed by elimination of water. The reagent is:
EASY
The product formed by the reaction of an aldehyde with a primary amine is:
HARD
In the reaction sequence
; the product B is:
MEDIUM
The major product of the following reaction is: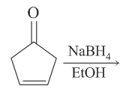
MEDIUM
In the following reactions
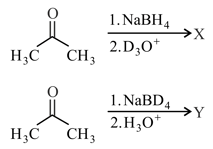
X and Y are
MEDIUM
The major product B formed in the following reaction sequence is:
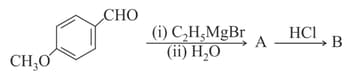
EASY
An organic compound 'X' having molecular formula yields phenyl hydrazone and gives negative response to the iodoform test and Tollen's test. It produces n-pentane on reduction. 'X' could be:
MEDIUM
The major product of the following reaction is:
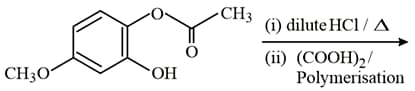
MEDIUM
Which of the following reagents would distinguish cis-cyclopenta-1, 2-diol from the trans-isomer?
HARD
The major product of the following reaction sequence is