Systematic Analysis of Cationic Radicals
Important Questions on Systematic Analysis of Cationic Radicals
In alkaline medium dimethyl glyoxime is used for the test of
An alkaline solution of potassium mercuric iodide is known as
Which gives blood red colour with ammonium thiocyanate?
A gas is obtained by addition of dil. to a mixture which turns lead acetate paper black. It is
For the given aqueous reactions, which of the statement(s) is (are) true?
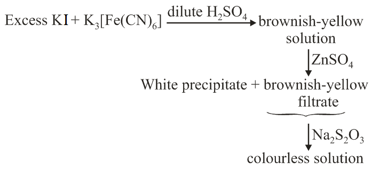
Upon treatment with ammoniacal , the metal ion that precipitates as a sulfide is
Which of the following sulphides are yellow?
Which pair of compounds is expected to show similar colour in aqueous medium?
Which of the following compound will not turn black on adding to it?
Out of the following is number of salt which have yellow or orange colour precipitates. Then value of is
Which among the following will be soluble in excess of
Solution of following cations is treated with excess of , the number of diamagnetic cations which form soluble complex is
The value of will be
Which of the following sulphates are soluble in water?
solution of aluminium chloride is used for the quantitative analysis of ions. If is added in a while ppt is formed and when excess is added it dissolved by forming soluble tetrahydroxoaluminate ion , if ions are added to , then
Tin (II) ion is used in identification of mercury (I) or mercury (II) ion in the qualitative analysis of basic radical. Select the correct statement about this experiment.
Experiment :
The number of intramolecular hydrogen bondings in complex
An aqueous solution of a mixture of two inorganic salts. When treated with dilute gave a precipitate of and a filtrate . The precipitate (P) was found to dissolve in hot water. The filtrate remained unchanged, when treated with in a dilute mineral acid medium. However, it gave a precipitate with ammonium chloride and ammonium hydroxide. The precipitate gave a coloured solution When treated with in an aqueous . The cation in precipitate and coloured solution contains respectively
The sulphides of some metal given in List I and their value is given in List II. What will be the correct option
| List-I | List-II | ||
| I | p | ||
| II | q | ||
| III | r | ||
| IV | s |
Generally chromium (III) is identified after oxidation to chromate ion. The oxidising agent that can be used for this oxidation is
Which of the following statements is not correct with reference to and ?

