Bond Parameters
Bond Parameters: Overview
This topic covers concepts such as bond parameters, bond length, bond enthalpy, bond order and crystal lattice formation of nacl.
Important Questions on Bond Parameters
Bond formation is :
In the compound,  , the bond is of the type –
, the bond is of the type –
How many among the given species have a bond order of
Arrange the following molecules according to decreasing order of their bond lengths.
Which pair among the following does not have the same bond order?
Determine the number of species having only two types of bond length.
In which of the following species bond angle decreases when all are replaced by -atoms.
Select the correct option regarding bond length.
Which of them has minimum value of where is bond angle of given species?
Which of the following has least bond angle = halogen?
The order of increasing bond length of is :
Which one of the following statements is correct for
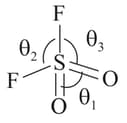
In which of the following bond is strongest among the given four compounds? central atom
The correct order in which the bond length increases in the following:
The correct order of decreasing bond angle is :
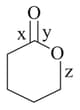 Correct order of Bond length is:
Correct order of Bond length is:
Correct order of bond lengths is :-
In how many of the following all bond lengths are not equal ?
white
In which of the following types of bond lengths are present :
The incorrect order of bond dissociation energy will be:-
