Crystal Field Theory (CFT)
Crystal Field Theory (CFT): Overview
This topic covers concepts, such as, Crystal Field Splitting Theory, Crystal Field Splitting in Tetrahedral Complexes, Conditions for Formation of Square Planar Complexes & Conditions for Formation of Tetrahedral Complexes etc.
Important Questions on Crystal Field Theory (CFT)
The value of the spin only magnetic moment for one of the following configurations is BM. The correct one is
A complex of metal has the following electronic distribution in orbitals,
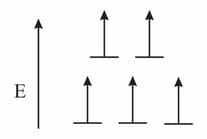
The neutral M has a ground state electronic configuration of Which of the following complexes is consistent with the electronic distribution of (as described above)?
In which of the following complexes, magnetic moment will change when all the ligands are replaced by ion to form a cyano complex?
In Wilkinson's catalyst, the hybridization of central metal ion and its shape are respectively:
An octahedral complex with magnetic moment may have:
(a) configuration with weak field ligand.
(b) configuration with strong field ligand.
(c) configuration with strong field ligand.
(d) configuration with weak field ligand.
Crystal field splitting energies for octahedral and tetrahedral geometries caused by the same ligands are related through the expression
The order of octahedral crystal field energy for orbitals are
According to crystal field theory, total number of unpaired electrons present in the following molecules and will be
An octahedral complex having configuration then correct for this complex is :
If the crystal filed splitting energy for octahedral complexes is and for tetrahedral complexes is , the two are related as
The crystal field-splitting for ion in octahedral field changes for and the increasing order is :-
The degeneracy of -orbitals is lost under :
(A) Strong field ligand
(B) Weak field ligand
(C) mixed field ligand
(D) Chelated ligand field
Choose the correct code :
In square planar complex of ion unpaired electrons are present in
Calculate crystal field stabilisation energy in :
The for complex is . The for will be ____.
The electronic configuration of central atom of complex is
The of octahedral is . The for tetrahedral will be :-
Predict the number of unpaired electrons in a square planar ion.
Which of following shows maximum value ?
Which order is correct in spectrochemical series of ligand ?
