Important Terms related to Coordination Compounds
Important Terms related to Coordination Compounds: Overview
This Topic covers sub-topics such as Coordination Number, Homoleptic Complexes, Heteroleptic Complexes, Coordination Sphere, Coordination Entity, Coordination Polyhedron, Counter Ion, Polynuclear Complexes, Perfect Complexes and, Imperfect Complexes
Important Questions on Important Terms related to Coordination Compounds
The coordination number of a central metal atom in the complex is determined by
Among the properties (a) reducing (b) oxidising (c) complexing, the set of properties shown by ion towards metal species is
What is called central atom?
What is coordination number?
Which of the following complex is a chelate complex?
Which complex compound is most stable?
Homoleptic complex from the following complexes is:
Number of ambidentate ligands in a representative metal complex is [ethylenediamine]
The set which does not have ambidentate ligand(s) is
Which of the following is not ambidentate ligand
Find out the oxidation number of central metal atom of ?
Calculate the sum of the oxidation states.
Define ligand and its types.
In the following complex ion , the oxidation state of iron is and is a polydentate ligand. Identify and name the ligand .
In 20th century, German scientist Werner succeeded in clarifying the structures of the five compounds consisting of platinum, chlorine, and ammonia. Some of the properties of these compounds are shown below in the table.
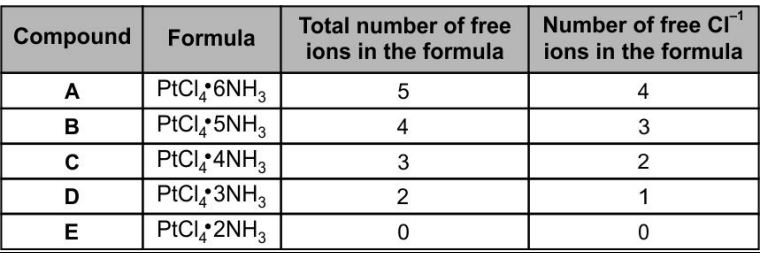
What is the oxidation state and coordination number of in compound ?
The compound is isomeric with the compound . Which of the following rows correctly represents the oxidation state of cobalt in these compounds?
| Rows | ||
A metal ion forms a complex ion of formula where represents a bidentate ligand. Which of the following could be the charge on the ligand ?
What are ligands? Predict whether the is chiral. Write structures of its enantiomers.
Oxidation number of the central metal atom in the compound is
why ambidentate ligand don't form two coordinate bonds at the same time or why ambidentate ligand behaves as a monodentate ligand?
Statement : contain bond.
Statement : Sulphur and Oxygen donate it's electron pair and act as ligand.
