Ellingham Diagrams
Ellingham Diagrams: Overview
This Topic covers sub-topics such as Ellingham Diagram, Limitations of Ellingham Diagram and, Applications of Ellingham Diagram
Important Questions on Ellingham Diagrams
Given below are two statements :
Statement I : According to the Ellingham diagram, any metal oxide with higher is more stable than the one with lower .
Statement II : The metal involved in the formation of oxide placed lower in the Ellingham diagram can reduce the oxide of a metal placed higher in the diagram.
In the light of the above statements, choose the most appropriate answer from the options given below
Given below are two statements.
Statement I: The choice of reducing agents for metals extraction can be made by using the Ellingham diagram, a plot of vs temperature.
Statement II: The value of increases from left to right in the Ellingham diagram.
In the light of the above statements, choose the most appropriate answer from the options given below:
The statement that is about Ellingham diagram is
In the Ellingham diagram for the solid metals and , gaseous oxides and are:
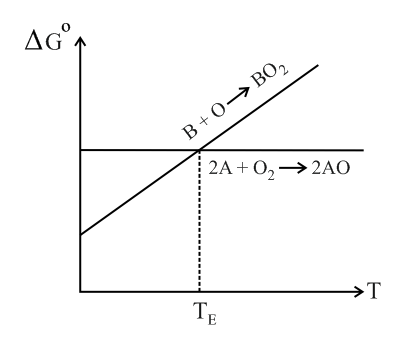
Below point can _____.
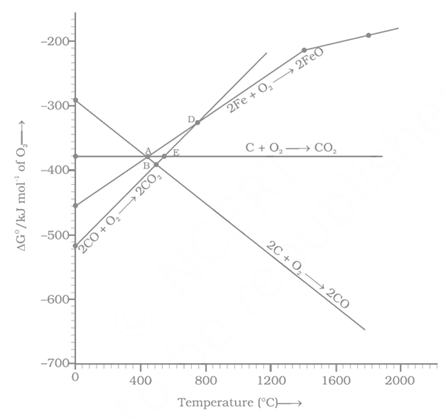
Which of the following metals cannot be obtained by reduction of its metal oxide by aluminium?
Ellingham diagram represents change of
Ellingham diagram is a graphical representation of :
Which one of the following statements is not correct?
Considering Ellingham diagram, which of the following metals can be used to reduce alumina?
vs plot in the Ellingham diagram sloper downwards for the reaction :-
In Ellingham diagram, the slope of curve of the formation of metal oxide :
Total number of plot in which slope is not correctly represented.
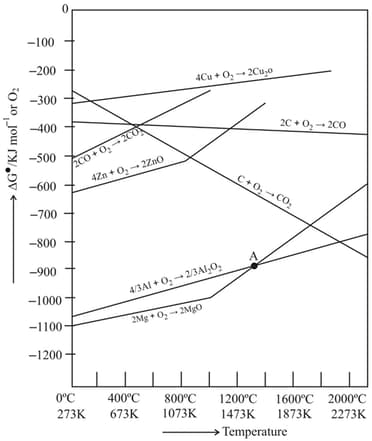
Explain the reason due to which Ellingham diagram does not explain the kinetics of the reduction process.
Explain limitations of Ellingham diagram based on the kinetics of the reaction.
What are the limitations of Ellingham's diagram.
Considering Ellingham diagram, which of the following metals can be used to reduce alumina?
Carbon cannot be used in the reduction of because:
To make the following reduction process spontaneous, temperature should be:
For a reaction
The free energy change is plotted as a function of temperature. The temperature below which the oxide is stable could be inferred from the plot as the point at which :
