Physical Properties of Amines
Physical Properties of Amines: Overview
This topic covers concepts such as Physical Properties of Amines, Hydrogen Bonding in Amines, Boiling Points of Amines, Water Solubility of Amines, and Colour and Odour of Amines.
Important Questions on Physical Properties of Amines
is soluble in which of the following compound?
Among the following molecules with the same molecular weight, which one has the highest boiling point?
Most soluble amine in aqueous solution is :-
Explain briefly about the odour of amines?
Explain briefly about the colour of aryl amines?
Arrange the following compounds in the decreasing order of their basic nature in gaseous phase. Ammonia, N - methylethanamine, propan - I - amine and N, N - dimethylmethanamine
Arrange the following compounds in the decreasing order of their boiling points
Ethylamine, n-propylamine, n-butylamineArrange the following compounds in the decreasing order of their boiling points
Ethane, ethylamine, and ethyl alcohol
Give plausible explanation for the statements. Butan - 1 - amine has higher boiling point than N - Ethylethanamine.
Give plausible explanation for the statements.-Butan - 1 - ol is more soluble in water than Butan - 1 - amine.
Give plausible explanation for the statement-Ethylamine is soluble in water whereas aniline is not.
Arrange the following compounds in the decreasing order of their solubility in water
ethylamine, diethylamine and triethylamine
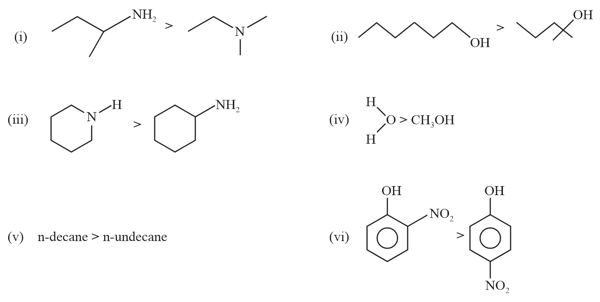
Among the following, how many are the correct order with respect to their Boiling points?
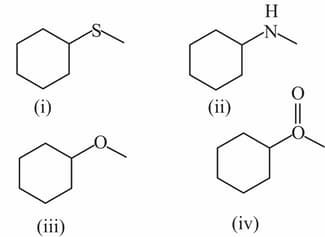
While trying to separate given compounds from their mixture, the mixture is treated with . Which of the following compound goes to aqueous layer ?
If A is is
Which of the following statement is correct about the basic strength on the basis of given data ?
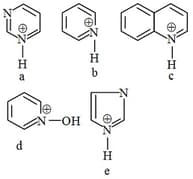
The correct pKa order is -
Which among the following has the highest boiling point?
Which among the following has the highest boiling point?
Which of the following amines form maximum hydrogen bonds within themselves?
