Stereochemical Aspects of Nucleophilic Substitution Reactions of Haloalkanes and Haloarenes
Stereochemical Aspects of Nucleophilic Substitution Reactions of Haloalkanes and Haloarenes: Overview
This Topic covers sub-topics such as Configuration, Racemic Mixture, Racemisation, Inversion of Configuration, Retention of Configuration, Chiral and Achiral, Dextrorotatory and Laevorotatory and, Molecular Asymmetry and Chirality
Important Questions on Stereochemical Aspects of Nucleophilic Substitution Reactions of Haloalkanes and Haloarenes
The structures shown here are related as being
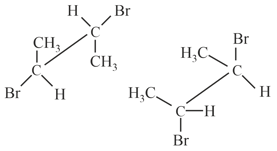
Which of the following structures represents a chiral compound?
What kind of reagent would be needed to resolve a racemic amine, such as 2-aminobutane?
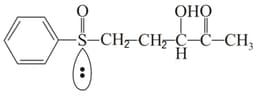
Number of chiral centers in the above molecule are:
A pure simple of -chlorobutane shows rotation of PPL by in standard conditions. When above samples is made impure by mixing its opposite form, so that the composition of the mixture become -form and -form, then what will be the observed rotation for the mixture.
What is stearic hindrance ?
The reaction which will not give chiral product(s) is :
Why are chiral carbons necessary for racemic mixture?
Observe the following compounds
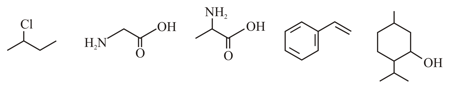
Fine the total number of structures/compounds which posses asymmetric carbon atoms.
How many optically active isomers are possible in the compound having formula ?
Which of the following compound is achiral?
In an reaction on chiral centres, there is:
Why nitrogen in penicillin is not chiral atom?
The number of optically active compound(s) obtained upon complete ozonolysis of the following optically active compound is
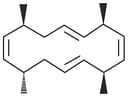
Relative configuration refers to the configuration of a molecule in relation to-
Define relative configuration
Consider the following compounds:
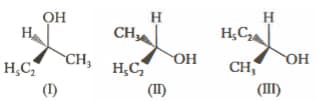
Which one of the following is correct in respect of the above compounds?
Explain relative configuration with the help of an example.
Define the term relative configuration.
Write difference between absolute and relatvie configuration.
