Fundamental Concepts in Organic Reaction Mechanism
Fundamental Concepts in Organic Reaction Mechanism: Overview
This topic covers concepts, such as, Organic Reactions and Mechanisms, Organic Reactions, Reactivity of Benzene & Orientation of Electrophile and Nucleophile in Benzyne etc.
Important Questions on Fundamental Concepts in Organic Reaction Mechanism
Which of the following has the highest nucleophilicity?
Which of the following is the strongest nucleophile?
Which of the followings is strongest nucleophile in ?
Which of the following is the strongest nucleophile?
Which of the following does not contribute to relative substrate specificity?
Which of the following is not an example of substrate?
Identify a weak nucleophile.
For the following reaction, identify the carbonyl compound that shows the highest reactivity.
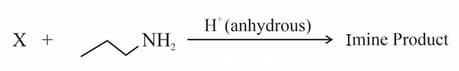
Which of the following is most electrophilic?
A sequential account of each step, describing details of electron movement, energetics during bond cleavage and bond formation, and the rates of transformation of reactants into products (kinetics) is referred to as _____ ?
_____ is that reactant which supplies carbon to the new bond and the other reactant is called reagent.
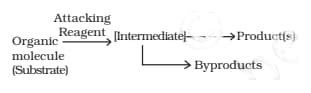
The above figure represents ______ ?
Weak bases such as neutral oxygens with a proton will be _____ ?
The reactions tends to proceed with _____ nucleophilies.
A molecule whose carbon is involved in new bond formation is called _____ ?
Aldehydes and ketones do not react with :
Which of the following poisonous gas caused Bhopal tragedy in ?
Which of the following statements is not correct for a nucleophile?
An organic reaction occurs through making and breaking of bonds. The breaking of bonds may occur either homolytically leading to the formation of radicals or heterolytically generating positively and negatively charged species. The neutral species (free radicals, carbenes, nitrenes, etc.) and positively charged species being electron deficient are collectively called electrophiles while neutral and negatively charged species which are electron rich are called nucleophiles. An organic reaction usually involves the attack of a reagent (radicals, positively and negatively charged species) on the substrate molecule). The substrate molecule, although as a whole electrically neutral, has centers of low and high electron density due to displacement of bonding electrons. These electron displacements occur through inductive, electromeric, resonance and hyperconjugation effects. Whereas inductive effects involve displacement -electrons, resonance effects involve transfer of - and -electrons along a conjugated system. Hyperconjugatlon effects, on the other hand, involve --conjugation. Both inductive and hyperconjugation effects can be used to explain the stability of carbocations and free radicals which follow the stabilIty order : . The stability of carbanions, however, follows opposite order.
The stability of a molecule can be judged on the basis of contribution of its resonance structures. Resonance structures have same position of nuclei and have the same number of unpaired electrons. Among resonance structures, the one which has greater number of covalent bonds, has less separation of opposite charges, a negative charge on more electronegative and a positive charge on a more electropositive atom are more stable than others.
Out of the following, the one containing only nucleophiles is
An organic reaction occurs through making and breaking of bonds. The breaking of bonds may occur either homolytically leading to the formation of radicals or heterolytically generating positively and negatively charged species. The neutral species (free radicals, carbenes, nitrenes, etc.) and positively charged species being electron deficient are collectively called electrophiles while neutral and negatively charged species which are electron rich are called nucleophiles. An organic reaction usually involves the attack of a reagent (radicals, positively and negatively charged species) on the substrate molecule). The substrate molecule, although as a whole electrically neutral, has centers of low and high electron density due to displacement of bonding electrons. These electron displacements occur through inductive, electromeric, resonance and hyperconjugation effects. Whereas inductive effects involve displacement -electrons, resonance effects involve transfer of - and -electrons along a conjugated system. Hyperconjugatlon effects, on the other hand, involve --conjugation. Both inductive and hyperconjugation effects can be used to explain the stability of carbocations and free radicals which follow the stabilIty order : . The stability of carbanions, however, follows opposite order.
The stability of a molecule can be judged on the basis of contribution of its resonance structures. Resonance structures have same position of nuclei and have the same number of unpaired electrons. Among resonance structures, the one which has greater number of covalent bonds, has less separation of opposite charges, a negative charge on more electronegative and a positive charge on a more electropositive atom are more stable than others.
Which of the following series contains only electrophiles?
