Arrhenius Collision Theory
Arrhenius Collision Theory: Overview
This topic covers concepts, such as, Effect of a Catalyst on Activation Energy of a Reaction, Graphical Representation of Rate Constant versus Temperature, Arrhenius Equation, Inhibitor & Intermediate Complex Theory etc.
Important Questions on Arrhenius Collision Theory
The rate of a reaction escalates four times when the temperature changes from to Determine the energy of activation of the reaction, assuming that it does not change with temperature?
The activation energy for the reaction is at . The fraction of molecules having energy equal to or greater than activation energy is:
For a decomposition reaction the values of rate constant k at two different temperatures are given below :
The value of activation energy for this reaction is:
For the adsorption of hydrogen on platinum, the activation energy is and for the adsorption of hydrogen on nickel, the activation energy is The logarithm of the ratio of the rates of chemisorption on equal areas of the metals at is (Nearest integer)
Given:
The correct reaction profile diagram for a positive catalyst reaction.
For the reaction under the same concentration conditions of the reactants, the rate of reaction at IS 1500 times as fast as the same reaction at . Calculate the activation energy of the reaction.If the frequency factor is Calculate the rate constant of the reaction at .
A bottle of milk stored at sours in . When stored in a refrigerator at it sours in Calculate the energy of activation of the reaction involved in the souring process. .
Give the nearest integer as the answer.
Among the following graphs showing variation of rate constant with temperature () for a reaction, the one that exhibits Arrhenius behaviour over the entire temperature range is
The rate constant of the first-order reaction, i.e., decomposition of ethylene oxide into and may be described by the following equation:
Find the energy of activation (in kJ/mole). Report answer till the nearest integer.
A certain physiologically important first-order reaction has activation energy equal to at normal body temperature Without a catalyst, the rate constant for the reaction is . To be effective in the human body, where the reaction is catalysed by an enzyme, the rate constant must be at least If the activation energy is the only factor affected by the presence of the enzyme, by how much must the enzyme lower the activation energy of the reaction to achieve the desired rate? (Report your answer by multiplying with 10 and rounding off to two significant figures)
A certain physiologically important first-order reaction has an activation energy equal to at normal body temperature Without a catalyst, the rate constant for the reaction is . To be effective in the human body, where the reaction is catalysed by an enzyme, the rate constant must be at least . If the activation energy is the only factor affected by the presence of the enzyme, by how much must the enzyme lower the activation energy of the reaction to achieve the desired rate?
Report the answer by multiplying the value with 10 and rounding off to two significant figures.
A certain physiologically important first-order reaction has activation energy equal to at a normal body temperature Without a catalyst, the rate constant for the reaction is . To be effective in the human body, where the reaction is catalysed by an enzyme, the rate constant must be at least . If the activation energy is the only factor affected by the presence of the enzyme, by how much must the enzyme lower the activation energy of the reaction to achieve the desired rate? Give answer to the nearest integer value after multiplying with 10.
The rate of a reaction decreased by times when the temperature was changed from to . The activation energy (in ) of the reaction is.........(Report the answer in the nearest integer value)
[Take; and ]
For the reaction, , the plot of vs is given below:
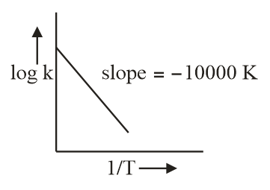
In the plot of a chemical process having and the slope is proportional to (where is equilibrium constant)
For a first order chemical reaction,
Which among the following equations represents Arrhenius equation ?
The activation energy for the forward reaction, is and the enthalpy change, of the reaction is . The activation energy (in ) for the backward reaction will be
Raw milk sours in hours at , but in hours in refrigerator at . What is activation energy (in ). for souring milk.
[Given
Round off your answer to the nearest integer.
Identify the statement which is correct from the following.
