Factors Influencing Rate of Reaction
Factors Influencing Rate of Reaction: Overview
This topic covers concepts, such as Factors Influencing Rate of a Chemical Reaction, Effect of Nature of Reactants on Rate of a Reaction, Effect of Concentration of Reactants on Rate of a Reaction, etc.
Important Questions on Factors Influencing Rate of Reaction
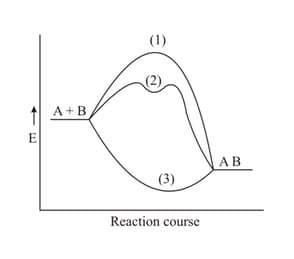
Which pathway does a catalyst-induced preparation of the substance follow if the reaction between is exothermic?
The hydrolysis of an ester was carried out separately with and . Which of the following expressions of rate constant is correct?
Which of the following statement is incorrect about the homogeneous reaction?
.
The rate a of reaction depends on which of the following factors?
Fill in the blanks in the following table for the reaction . The reaction is of first order w.r.t. X and zero order w.r.t. Y.
| Exp. | Initial rate | ||
|---|---|---|---|
The rate of a gaseous reaction is given by the expression . The volume of the reaction vessel is reduced to one half of the initial volume. What will be the reaction rate as compared to the original rate 'a' ?
When a chemical reaction (of order one or more) takes place, during the course of the reaction the rate of reaction
The rate of a chemical reaction involving liquids only will not be influenced by the
Suppose is the intensity of absorbed light and concentration of is , then in the photochemical excitation of , the rate of excitation of is proportional to
The rate constant for the reaction, is . If the rate of reaction is , then concentration of is-
For an elementary reaction:
If the volume of the reaction vessel is reduced to one-third of its original volume, what will be the new rate of the reaction?
The reaction is found to be first order in A, second in B and zero order in C. What is the effect on the rate of increasing concentration of A, B and C two times?
For a reaction between gaseous compounds,
The reaction rate=k[A][B]. If the volume of the container is made of the initial, then what will be the rate of reaction as compared to the initial rate?
Rate of reaction depends upon
Pieces or chunks of wood burn faster than a log of wood of the same mass because
For the reaction system, , volume is suddenly reduced to half its value by increasing the pressure on it. If the reaction is of first-order concerning and second-order concerning , the rate of reaction will
Rate of reaction depends upon
The phenomenon of emission of visible light as a result of chemical change is known as:
Which of these does not influence the rate of reaction?
Rate of reaction depends upon:
