Batteries
Batteries: Overview
This topic covers concepts, such as, Hydrogen-Oxygen Fuel Cell, Advantage of Hydrogen-Oxygen Fuel Cell, Nickel-Cadmium Storage Cell & Calculation of Percentage Efficiency of a Fuel Cell etc.
Important Questions on Batteries
The type of cell in a lead storage battery is:
The conductance of metallic substance is because of
The equilibrium constant for the reaction would be:
Given thatDuring the discharge of a lead storage battery, the density of sulphuric acid fell from 1.294 to 1.139 Sulphuric acid of density 1.294 by weight and that of 1.139 by weight. The battery holds 3.5 litres of the acid and the volume remained practically constant during the discharge.
The number of ampere-hours for which the battery must have been used. The charging and discharging reactions are:
Anode:
Cathode:
Note: Both the reactions take place at the anode and cathode respectively during discharge. Both reaction get reverse during charging.
A cell, initially contains 1 M and 1 ions.
Calculate the change in the cell potential after the passage of 9.65 A of current for 1 h.
For the electrochemical cell,
From this data one can deduce that:
In hydrogen-oxygen fuel cell, combustion of hydrogen occurs to –
Button cells are used in portable electronic devices.
In the silver oxide button cell, zinc act as a cathode.
The biggest difference between the two is that a battery stores energy, while a fuel cell generates energy by converting available _____.
Which of the following is correct labelling of lead storage battery:
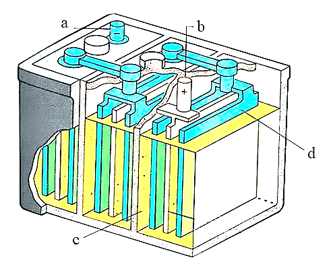
In hydrogen-oxygen fuel cell, the carbon rods are immersed in hot aqueous solution of:
Which of the following are correctly label for fuel cell
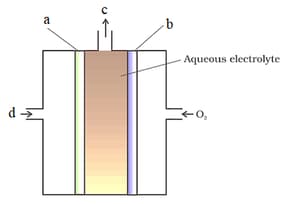
Which of the following is correct labelling of fuel cell:
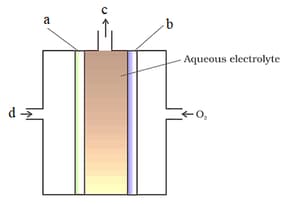
Galvanic cells that are designed to convert the energy of combustion of fuels like hydrogen, methane, methanol, etc. directly into electrical energy are called fuel cells.
The anode reaction which is taking place in nickel- cadmium battery can be represented by which of the following equation?
Define fuel cell and write its two advantages.
Cathode is made of ...... in mercury battery :-
