Indicators and Acid-base Titrations
Indicators and Acid-base Titrations: Overview
This topic covers concepts, such as, Acid-base Indicators, Ostwald's Theory of Acid-base Indicators, The Titration of a Weak Base with a Weak Acid & Titration of Strong Base with a Weak Acid etc.
Important Questions on Indicators and Acid-base Titrations
-methyl benzene sulphonic acid reacts with sodium acetate to give
When a weak acid is titrated with a weak base, what is the At the equivalence point?
Is Phenolphthalein and methyl orange weak acid and weak base explain it?
According to Ostwald's theory of acid-base indicators, the colour change in titration is due to the ionisation of the acid-base indicator.
According to quinonoid theory, the acid-base indicators exist in two tautomeric forms having different structures.
Explain the quinonoid theory of acid-base indicators.
Phenolphthalein does not act as an indicator for the titration between:
The indicator used in the titration of sodium carbonate with sulphuric acid is:
The suitable indicator for strong acid and weak base is:
Which is/are correct statements?
(i) In any strong acid solution, the concentration of will be zero.
(ii) If of a reaction is positive, then the reaction will not proceed at all, in the forward direction for any concentrations of reactants and products.
(iii) Titration curves are drawn for (about the figure shown).
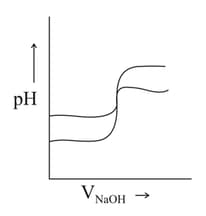
(a) with and(b) with on the same graph paper they look like
The pink colour is discharged when the compound, is mixed with a dilute solution of containing a drop of phenolphthalein and boiled. The compound could be
Consider x mL of HCl is used when phenolphthalein is used as an indicator and y mL of HCl is used when methyl orange is the indicator in two separate titrations of 20 mL of 0.1 M solution of compound Na2CO3.NaHCO3.2H2O which is titrated against 0.05 M HCl. Hence (y - x) is :
What is the volume of hydrogen chloride(HCl) required for complete reaction of sodium bicarbonate(NaHCO3) in a double titration method, for the mixture of sodium bicarbonate(NaHCO3) and sodium carbonate (Na2CO3).If the volume of hydrogen chloride(HCl) used at phenolphthalein indicator end point was x ml and further of Methyl orange indicator end point was y ml.
What is the volume of HCl required for complete neutralization of NaHCO3 .In the given mixture of Na2CO3 and NaHCO3 , if x mL of HCl required with phenolphthalein indicator and y mL of HCl required with methyl orange indicator for neutralization of the mixture.
Among the following acids, which acid has a smallest value of dissociation constant?
In which case phenolphthalein will not be effective as an indicator?
For the indicator HIn the ratio is 7.0 at pH of 4.3.
Keq for the indicator is:
[Given log 7 = 0.845 and Antilog(0.545)=3.5
Cotyledons are also called-
An alkali is titrated against an acid with methyl orange as indicator, which of the following is a correct combination?
The table below gives the results of three titrations carried out with 0.200 M HCl to determine the molarity of a given NaOH solution using phenolphthalein as indicator. NaOH was taken in the burette and HCl was taken in a conical flask for the titrations
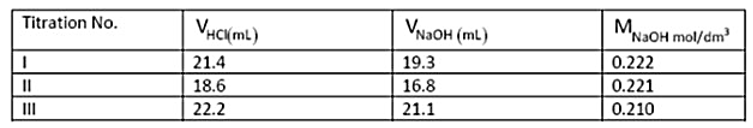
The actual molarity of the prepared NaOH solution was 0.220 mol dm-3. Which among the following could be the reason for the wrong value obtained in titration III?
