Packing Efficiency in Solids (2D or 3D)
Packing Efficiency in Solids (2D or 3D): Overview
This topic covers concepts, such as, Packing Efficiency in CCP and HCP Structures, Packing Efficiency in BCC Structures, Packing Efficiency in Simple Cubic Lattice & Packing Fraction of an Unit Cell etc.
Important Questions on Packing Efficiency in Solids (2D or 3D)
A metallic element crystallises into lattice having a layering sequence of Any packing of sphere leaves out voids in the lattice. Determine what percentage by volume of this lattice is empty space.
In curing cement plasters water is sprinkled from time to time. This helps in
Atom occupies the fcc lattice sites as well as alternate tetrahedral voids of the same lattice. The packing efficiency (in ) of the resultant solid is closest to
The packing efficiency in a body centered cubic (bcc) structure is closest to
If the packing fraction for spheres occupying a body-centred cubic structure is X, Report your answer by multiplying X with 100
Calculate the percentage of vacant space in a unit cubic cell. The unit-cell content for is and .
The packing fraction of the element that crystallizes in simple cubic arrangement is -
Packing efficiency in simple cubic unit cell is (in percentage):
Packing efficiency in FCC unit cell is(in percentage):
The percentage of empty space in a body-centred cubic arrangement is.
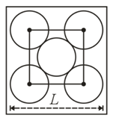
The packing efficiency of the two-dimensional square unit cell shown below is :
Total volume of atoms present in a unit cell of a metal is:-
The maximum packing efficiency of an ionic solid, (fluorite structure) is about
In which pair most efficient packing in present?
Percentage of free space in cubic close-packed structure and in body centered packed structure are respectively :
The fraction of total volume occupied by the atoms present in a simple cube is
A crystal lattice is made up of a very large number of unit cells where every lattice point is occupied by one constituent particle. The unit cell can be seen as a three dimension structure containing one or more atoms. We always observe some void spaces in the unit cell irrespective of the type of packing. Percentage of spaces filled by the particles in the unit cell is known as the packing fraction of the unit cell. In this section we shall learn about packing efficiency.
The packing fraction for a body centred cubic structure is
of has atoms arranged in cubic packing to give the best packing efficiency. The possible arrangement is
Percentages of free space in cubic close-packed structure and in the body-centered packed structure are respectively.
The vacant space in unit cell is
