Adsorption
Adsorption: Overview
This topic covers concepts, such as, Adsorption, Adsorbent, Heat of Adsorption & Adsorption from Solution Phase etc.
Important Questions on Adsorption
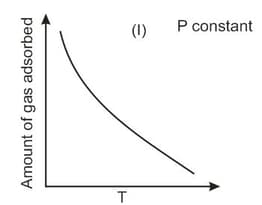
The given graphs/data I, II, III and IV represent general trends observed for different physisorption and chemisorption processes under mild conditions of temperature and pressure. Which of the following choice(s) about I, II, III and IV is (are) correct?
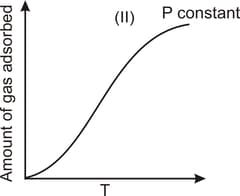
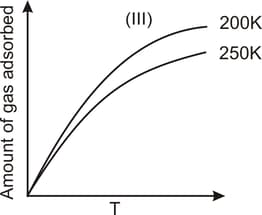
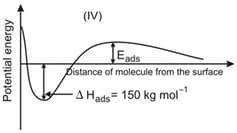
Among physisorption and chemisorption, which type of a adsorption has a higher enthalpy of adsorption?
20% of surface sites are occupied by molecules. The density of surface sites is and total surface area is . The catalyst is heated to 300 K while is completely desorbed into a pressure of 0.001 atm and volume of . The number of active sites occupied by each molecule will be
Adsorption of gases on solid surface is generally exothermic because:
Predict the signs for entropy and enthalpy of adsorption.
Why unimolecular layer is formed in chemical adsorption?
How many layers are adsorbed in chemical adsorption?
Pressure has no effect on chemical adsorption.
How long animal charcoal retains its absorbent properties?
What is the difference between charcoal and activated charcoal?
The molecular species or substance, which concentrates or accumulates at the surface is termed adsorbate.
The molecular species or substance, which concentrates or accumulates at the surface is termed _____.