Specific Heat Capacity and Molar Heat Capacity
Specific Heat Capacity and Molar Heat Capacity: Overview
This topic consists of various concepts like Specific Heat Capacity,Heat Capacity,Molar Specific Heat Capacity, etc.
Important Questions on Specific Heat Capacity and Molar Heat Capacity
The molar heat capacity of water at constant pressure is . When 1kJ of heat is supplied to 100g of water, which is free to expand, the increase in temperature of water is
A bullet of mass is fired with velocity and stop after hitting the target. Ifof its . goes increasing the temperature of the bullet then find raise in temperature (Specific heat capacity of bullet is )
A body of mass falls from a height of and rebounds to a height of . If the loss in energy goes into heating the body, then the rise in temperature will be nearly: (specific heat of material is )
Below is a graph between change in internal energy and temperature for a system placed in a constant volume.
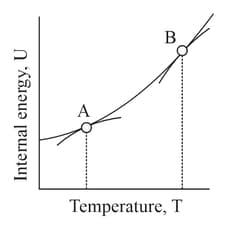
The slope of the graph is known as
Which one of the following material will expand maximum if the same amount of heat energy is given to them?
of energy supplied to of water will raise its temperature by nearly
A block of specific heat capacity is heated with a heater of power at efficiency . Find the change in temperature if mass of the block is .
Two beakers and contain liquids of mases and respectively and specific heats and . The amount of heat on them is equal. If they are joined by a metal rod
Three identical silver cups and contain three liquids of same densities at same temperature higher than the temperature of the surrounding. If the ratio of their specific heat capacities is , then
How much heat energy is necessary to raise the temperature of of ice from to steam at ?
If the heat given to raise the temperature of two solid spheres of radius and and density and through is same, then the ratio of their specific heat capacity is:
In a certain system of units, the fundamental unit of mass is taken as unit of length is taken as unit of time taken as and unit of temperature is taken as Kelvin. In this system. unit of specific heat capacity will be (specific heat capacity is given by Where is heat, is mass and is change in temperature) :-
Two identical systems, with heat capacity at constant volume that varies as (where is a constant) are thermally isolated. Initially, one system is at a temperature and the other is at . The systems are then brought to thermal contact and the combined system is allowed to reach thermal equilibrium. The final temperature (in ) of the combined system is . Write the value of where is the greatest integer function.
During an adiabatic process, the pressure of a gas is found to be proportional to the cube of its absolute temperature. The ratio for the gas is . Find .
What is the relationship between heat and temperature?
Define specific heat capacity with unit.
Define heat capacity with unit.
If calories of heat are supplied to of water, its temperature rises from to If specific heat for water is , then the value ofis
Equal masses of three liquids have temperatures respectively. If are mixed, the mixture has a temperature of . If are mixed, the mixture has a temperature of . If are mixed, then the mixture will have a temperature in
Define specific heat of an object. Write the expressions for heat energy absorbed and lost by an object in terms of specific heat. Explain the process of measurement of specific heat by mixing method in a calorimeter.
