Heat, Internal Energy and Work
Heat, Internal Energy and Work: Overview
This topic consists of various concepts like Internal Energy,Work Done by Thermodynamic System,Internal Energy in Thermodynamics, etc.
Important Questions on Heat, Internal Energy and Work
When work done by a system was , the increase in the internal energy of the system was . The heat 'q' supplied to the system was
Consider the following statements-
a) is a state function in isochoric process.
b) is state function in isobaric process.
c) Work done by the system will be zero in adiabatic free expansion.
Correct among the following is/are
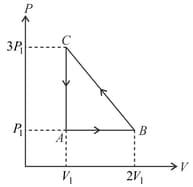
The net work done by an ideal gas going through the cycle as shown in the diagram below is
The internal energy of an ideal gas depends on
A gas mixture consist of mole of hydrogen and mole of Helium at absolute temperature . Considering all vibrational modes, (assume zero internal energy at ) the total internal energy of the system is
A monatomic gas (ideal) is supplied joule heat at constant pressure. The internal energy of gas, increases by
Is mass related to internal energy in thermodynamics?
Two moles of an ideal non-linear triatomic gas undergo a transition from to along a path as shown in the figure. Change in internal energy of the gas is
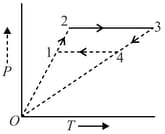
Three moles of an ideal monatomic gas perform a cycle as shown in figure. The gas temperatures in different states are . Find the work done by the gas during the cycle in : (use )
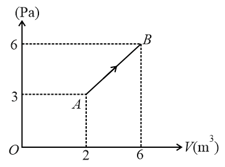
A gas mixture consists of of oxygen and of argon at temperature . Neglecting all vibrational modes, the total internal energy of the system is . Find .
One mole of a monoatomic gas is taken from to , via three paths and . If work done by the gas in is , in work done is and in work done is , then
Distinguish between internal energy and heat.
What is the difference between heat and internal energy?
Define work done in a thermodynamic process.
An ideal monoatomic gas is confined in a horizontal cylinder by a spring loading piston (as shown in Fig ). Initially the gas is at temperature pressure and volume and the spring is in relaxed state. The gas is then Fig. heated very slowly to temperature , pressure and volume . During this process the piston moves out by a distance . Ignoring the friction between the piston and the cylinder, the correct statements is (are)
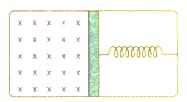
Which part of the solar cooker is responsible for the green house effect?
What is the distinction between heat and internal energy?
An ideal is filled in a closed, rigid and thermally-insulated container. A coil of resistance and carrying a current of supplies heaT to the gas. The change in the internal energy of the gas after minutes will be:
For an ideal gas, its internal energy is the function of its:
Write the difference between.heat and internal energy
